CDSCO LICENSE: APPLY ONLINE CDSCO REGISTRATION
The Central Drugs Standard Control Organization (CDSCO) serves as the primary regulatory authority for pharmaceuticals and medical devices in India. Operating under the Directorate General of Health Services, Ministry of Health and Family Welfare, CDSCO holds the responsibility of approving new drugs and overseeing clinical trials in accordance with the Drugs and Cosmetics Act, 1940 ("Act") and Drugs and Cosmetics Rules, 1945 ("Rules"). CDSCO plays a crucial role in regulating aspects related to drugs and cosmetics to ensure the safety, rights, and well-being of patients. It also supervises the efficiency and quality of medical products manufactured, imported, and distributed within the country.
Under the Act and Rules, both Central and State Regulators are given specific duties for regulating drugs and cosmetics in India.
CDSCO functions as the Central Drug Authority, carrying out the tasks assigned to the Central Government under the Act. It provides expert guidance to State Drug Control Organizations and collaborates with them. CDSCO, along with state regulators, shares the responsibility of granting licenses for certain specialized categories of critical drugs like vaccines, blood products, IV fluids, and sera.
Any organization involved in importing, manufacturing, or exporting drugs and cosmetics, conducting R&D activities related to drugs, importing drugs for testing, or conducting Bioavailability (BA) and Bioequivalence (BE) studies in drugs for export purposes is required to obtain registration under CDSCO through its registration portal. The CDSCO online registration portal simplifies the registration process for medical devices, drugs, cosmetics etc. CDSCO registration for medical devices requires a thorough understanding of the process.
When a product has a CDSCO Certificate, it means the item has gone through tough testing, followed all the rules, and has been thoroughly checked for how it's made, what it's made of, and how it's packaged. This ensures there are no cutting corners or taking chances. The CDSCO license registration certificate is proof of compliance with regulations.
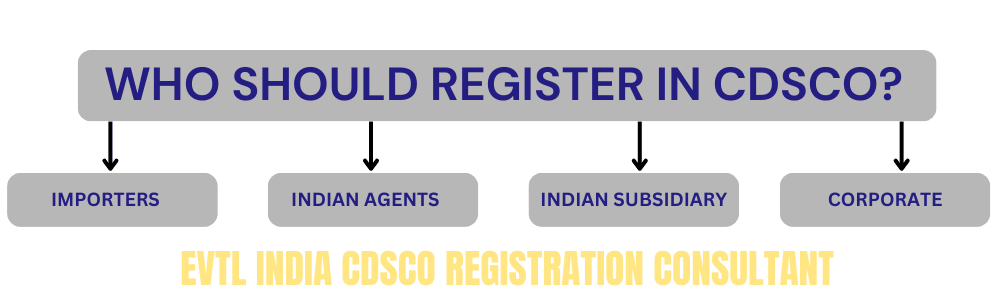
WHO IS ELIGIBLE TO REGISTER WITH CDSCO?
The CDSCO online portal allows the following entities to register:
Importers
Indian Agents
Foreign Enterprises with an Indian Subsidiary
Corporate
Note: Manufacturing units cannot directly register on the portal. Instead, a corporate entity must create login credentials for the manufacturing unit to access the portal.
WHAT ARE THE DIFFERENT SECTIONS OF CDSCO?
CDSCO in India has various sections that deal with different aspects:
Cosmetics: According to the Drugs & Cosmetics Act, cosmetics include items intentionally applied to the human body for decoration, cleaning, glowing, or changing appearance. Manufacturing of cosmetics is regulated by State Licensing Authorities, and import is governed under a registration system by the Central Government.
DCC-DTAB: This division provides recommendations on matters related to the administration of the Act and Rules. It organizes meetings to effectively monitor and update rules.
BA/BE (Bioavailability/Bioequivalence): BA refers to the amount of a drug that reaches the bloodstream, while BE means two drugs function the same. These aspects are crucial for drug effectiveness.
Import and Registration of Drugs: Import of drugs into India is regulated under the Act and Rules. Applications for registration and import licenses are processed according to the Drugs & Cosmetics Rules.
New Drugs: Safety and efficacy evidence is required for new drugs before manufacturing or import. New drugs include substances not extensively used in India and not recognized as safe by the authority.
Medical Devices and Diagnostics: Only notified medical devices are regulated as drugs under the under Act & Rules of Drugs & Cosmetics. This includes various items like substances for artificial insemination, surgical dressings, prophylactic techniques, biological products, and vaccines.
Biological: Biological products, made from natural sources, are used to treat diseases or prevent/diagnose them. This section covers gene and cellular therapies, blood donor tests, allergic extracts, and more.
Vaccines: This section includes things like using human organs, cells, or tissues for transplantation, gene and cellular therapies, tests for blood donors, and extracts from plants that are used for both treatment and diagnosis.
BENEFITS OF CDSCO REGISTRATION
Legal Compliance: CDSCO Registration ensures that the production and sale of drugs and cosmetic products follow all the necessary laws and regulations.
Product Safety: CDSCO ensures that registered cosmetic products are free from harmful substances, making them safe for public use.
Quality Assurance: The registration process guarantees that cosmetic products meet quality standards, ensuring they are suitable for widespread use.
Market Accessibility: Before entering the market, CDSCO Registration is mandatory, ensuring that products are safe and toxin-free, opening up new opportunities for businesses.
Consumer Protection: By verifying products for safety and quality, CDSCO safeguards the well-being of consumers, assuring them that registered items are safe to use.
CDSCO REGISTRATION PROCESS:
To register on the CDSCO portal, follow these online steps:
- Visit the CDSCO registration portal and click 'Login/Sign Up.'
- Choose 'Sign Up Here' and select the registration purpose.
- Fill in applicant details, including username, password, name, mobile number, and email ID.
- Upload ID proof details and an undertaking, and provide registered Indian address details.
- Click 'Submit,' and a confirmation link will be sent to your email for verification.
- Activate your account by clicking the link in the email.
- The application is sent to CDSCO officials for approval.
- Upon approval, you'll receive an email confirmation, completing the registration process.
DOCUMENTS REQUIRED FOR REGISTRATION UNDER CDSCO
To register on the CDSCO portal, you need to submit certain documents along with your application:
- ID Proof Document
- Undertaking issued by a Government Authority
- Address Proof Document
- Copy of BA/BE Site Registration as approved by CDSCO in case of BA/BE Approved sites Registration
- Manufacturing License or Wholesale Licenses in case of Import or Manufacture of Drugs/Blood Product Registration/Test license Registration
CONCLUSION
CDSCO establishes standards for drugs, monitors the quality of imported drugs and cosmetics, and aims to maintain consistency in enforcing the Act and Rules. A CDSCO Certificate is like an official approval given by the authorities who make sure those medicines, medical devices, and cosmetics work well and are of good quality.
To import medical devices, obtaining a CDSCO import license is crucial. For drugs, the drag license and CDSCO registration are mandatory for legal operations. The CDSCO registration process is essential for acquiring a medical device manufacturing license. Medical license and CDSCO license registration are prerequisites for selling pharmaceuticals.
Navigating the CDSCO Sugam portal is part of the medical device registration process. A CDSCO license consultant can provide guidance on the registration process and fees. For consultancy, Axis Compliance is here to assist you. Contact us via email at contact@axiscomplience.in or give us a call at +91 8178824481 The CDSCO registration fees vary based on factors such as the type of license and product. Acquiring a CDSCO license involves adhering to specific guidelines for product registration. Medical license holders must comply with CDSCO regulations to maintain their status.
- Home
- About Us
-
Services
- BIS ISI Mark Certification
- BIS-CRS Certification
- ISI Domestic Manufacture
- EPR Plastic Waste
- EPR E-Waste
- EPR Registration
- EPR Battery Waste
- BIS FMCS Registration
- WMI Registration
- BIS ECO Mark Scheme
- BIS Certification for Footwear
- EMI-EMC Test
- RF Testing
- IP Rating Test
- TEC Approvals
- NABL Testing
- LM 79 & LM 80
- ROHS Approval
- CE Certificetion
- EPR Importance
- EPR For Tyres
- EPR For Used Oil
- TradeMark
- Copy Right
- WPC-ETA Approval
- BEE Registration
- FSSAI Registration
- Gem Registration
- BIS Certification for Toys
- Import Export License
- Custom Compliance
- LAB Setup and lab equipment
- UL Certification
- CDSCO Approvals
- Drug License
- NOC For Steel
- IMEI Registration
- ISO Certification
- Legal Metrology
- NSIC Registration
- Start-Up Registration
- Make in India Mark
- LMPC Registration
- CDSCO Registration
- Updates
- Gallery
- Clients
- Contact Us